Lessico
Granato

Nome generico di un gruppo di minerali accomunati dallo stesso sistema di cristallizzazione, spesso utilizzati come gemme o come abrasivi. I granati cristallizzano nel sistema cubico, di solito come rombododecaedri, icositetraedri o combinazioni delle due forme.
Le diverse varietà di granato mostrano quasi tutte le colorazioni, con l'eccezione del blu; i colori più comuni sono il bruno, il rosso, il giallo, il nero; sono frequenti anche i granati incolore. Le pietre più scure sono di solito opache, mentre quelle chiare possono essere trasparenti o traslucide. La durezza del granato varia da 6 a 7,5, e la densità da 3,6 a 4,3. I granati sono tutti silicati, e le diverse varietà si distinguono per la composizione chimica. Le formule qui riportate sono approssimate, perché da un esemplare all'altro le proporzioni degli elementi sono raramente le stesse.
Granato
Il termine granato designa un gruppo di minerali nesosilicati, di cui alcuni
in uso fin dall’età del bronzo come gemme; trovare granati nei gioielli
degli antichi egiziani, greci e romani non è raro. Il nome
"granato" deriva dal latino granatus (grano), con un
probabile riferimento al malum granatum (melograno![]() ), pianta
con semi rossi con forma e colore simili a quelli di alcuni cristalli di
granato.
), pianta
con semi rossi con forma e colore simili a quelli di alcuni cristalli di
granato.
È diffusa l’erronea credenza che il granato sia una pietra preziosa rossa: in realtà esso si presenta in una serie di colori che va dal viola, arancione, rosa, giallo, verde, marrone e nero all’incolore, sebbene con il termine generico "granato" ci si riferisca di solito alla varietà rossa piropo. Le varietà principali dei granati sono sei, raggruppate in due serie isomorfe: piralspite (piropo-almandino-spessartina) e ugrandite (uvarovite-grossularia-andradite).
Aspetto
I granati si presentano in vari colori tra cui il rosso, arancione, giallo, verde, rosa, viola, marrone, nero e blu, il più raro in natura. Vi sono anche diverse varietà di granati che cambiano colore al variare della luce a cui sono esposti (naturale o a incandescenza). I granati possono avere una lucentezza che ne fa belle pietre preziose oppure essere opachi e quindi utilizzati a scopi industriali come agenti abrasivi.
Struttura cristallina
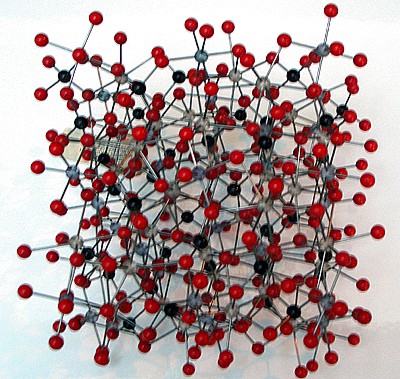
Modello molecolare del granato
I granati sono nesosilicati con formula chimica generale X3Y2(SiO4)3. Il sito X è di solito occupato da un catione bivalente (Ca2+, Mg2+, Fe2+) e il sito Y da cationi trivalenti (Al3+, Fe3+, Cr3+) in una struttura ottaedrica o tetraedrica. L’abito cristallino è prevalentemente dodecaedrico e trapezoedrico, e il sistema cristallino è cubico.
Granati alluminosi - Questa serie di granati viene chiamata anche con l'acronimo piralspite e comprende i seguenti minerali:
Almandino - Fe3Al2(SiO4)3
Piropo - Mg3Al2(SiO4)3
Rodolite - (Mg,Fe)3Al2(SiO4)3
Spessartina - Mn3Al2(SiO4)3

Almandino
Almandino - L’almandino, di colore rosso, è la varietà più comune di granato, ed è spesso utilizzata come pietra preziosa, conosciuta anche come granato orientale o rubino almandino. Esso è presente per lo più nelle rocce metamorfiche, in particolare negli scisti e nei metascisti.
Piropo - Il piropo, come dice il nome, ha tipicamente colore rosso fuoco, e per questo gli esemplari trasparenti, anche se meno comuni dell’almandino, sono spesso stati usati come gemme nel XVIII e XIX secolo, e ancora oggi, col nome di rubino del Capo, rubino dell’Arizona e granato boemo. Il piropo si trova nelle rocce peridotite e eclogite, e di esso esiste una variante rosa-violetta ritrovata nella Carolina del Nord chiamata Rodolite e un’altra molto rara, scoperta nel 1990 Madagascar, che è frutto della mistura con la spessartite e ha un colore blu cangiante.
Famosi per la loro purezza (95-98%) e per la dimensione (fino a 20kg) gli esemplari di Piropo di Martiniana Po (CN). Proprio a causa delle loro straordinarie misure sono stati per molto tempo considerati delle "curiose pietre arrotondate, finché nel 1984 il professor Chopen ne identificò la natura e l'origine. Pare si siano formati a oltre 100 km di profondità, per poi emergere a causa dei movimenti tettonici. Il colore è rosato e la loro formula rispecchia "quasi perfettamente" quella del piropo; al loro interno risultano inclusi altri rari minerali quali ellebergite, magnesiodumortierite, bearthite, coesite e rutilo. Dato l'elevato valore scientifico, la zona dei ritrovamenti è interdetta alla ricerca se non con l'autorizzazione di istituti scientifici.

Spessartina
Spessartina - La spessartina ha colore giallo-arancione e, sebbene abbastanza rara, è conosciuta e utilizzata come gemma a scopi commerciali col nome di granato mandarino. È presente nei graniti permatiti e in certi filliti all’inizio del loro processo metamorfico.
Granati calcici - Questa serie di granati viene chiamata anche con l'acronimo ugrandite e comprende i seguenti minerali:
Andradite - Ca3Fe2(SiO4)3
Demantoide - Ca3Fe2(SiO4)3
Melanite - CaFeTi(SiO4)3
Topazolite - Ca3Fe23+(SiO4)3
Grossularia - Ca3Al2(SiO4)3
Essonite - Ca3Al2(SiO4)3
Tsavorite - Ca3Al2(SiO4)3
Uvarovite - Ca3Cr2(SiO4)3

Andradite
Andradite - L’andradite può avere colore nero, verde o giallo-marrone, in base a quale delle tre varietà è presa in considerazione: melanite, demantoide (il granato forse più raro e prezioso, a parte la variante blu del piropo) e topazolite, di qualità a volte sufficiente per l’uso come pietra preziosa. L’andradite si trova in rocce magmatiche come le sieniti e nei serpentini, scisti e calcare cristallino.
Grossularia - La grossularia, di cui vi sono due varietà, essonite e tsavorite (molto ricercata ed usata come pietra preziosa), è di colore rispettivamente rosso e verde, ma vi sono anche esemplari gialli-marroni e incolori. Si può reperire nelle rocce metamorfiche generate per metamorfismo di contatto di sedimenti calcarei e di vesuvianite, diopside, wollastonite e scapolite.

Uvarovite
Uvarovite - L’uvarovite, di colore verde smeraldo, è tra j granati più rari e ricercati ed è rintracciabile principalmente nei serpentini.
Garnet is a group of minerals that have been used since the Bronze Age as gemstones and abrasives. Garnets are most often seen in red, but are available in a wide variety of colors spanning the entire spectrum. The name "garnet" comes from the Latin granatus (grain), possibly a reference to the Punica granatum (pomegranate), a plant with red seeds similar in shape, size, and color to some garnet crystals.
Six common species of garnet are recognized based on their chemical composition. They are pyrope, almandine, spessartite, grossular (varieties of which are hessonite or cinnamon-stone and tsavorite), uvarovite and andradite. The garnets make up two solid solution series; 1. pyrope-almandine-spessarite and 2. uvarovite-grossular-andradite.
Properties
Garnets species are found in many colors including red, orange, yellow, green,
blue, purple, brown, black, pink and colorless. The rarest of these is the
blue garnet, discovered in the late 1990s in Bekily, Madagascar. It is also
found in parts of the United States, Russia and Turkey. It changes color from
blue-green in the daylight to purple in incandescent light, as a result of the
relatively high amounts of vanadium (about 1 wt.% V2O3). Other varieties of
color-changing garnets exist. In daylight, their color ranges from shades of
green, beige, brown, gray, and blue, but in incandescent light, they appear a
reddish or purplish/pink color. Because of their color changing quality, this
kind of garnet is often mistaken for Alexandrite.
Garnet species’s light transmission properties can range from the
gemstone-quality transparent specimens to the opaque varieties used for
industrial purposes as abrasives. The mineral’s luster is categorized as
vitreous (glass-like) or resinous (amber-like).
Crystal structure
Garnets are nesosilicates having the general formula X3Y2(SiO4)3. The X site is usually occupied by divalent cations (Ca2+, Mg2+, Fe2+) and the Y site by trivalent cations (Al3+, Fe3+, Cr3+) in an octahedral/tetrahedral framework with [SiO4]4- providing the tetrahedra. Garnets are most often found in the dodecahedral crystal habit, but are also commonly found in the trapezohedron habit. (Note: the word "trapezohedron" as used here and in most mineral texts refers to the shape called a Deltoidal icositetrahedron in solid geometry.) They crystallize in the cubic system, having three axes that are all of equal length and perpendicular to each other. Garnets do not show cleavage, so when they fracture under stress, sharp irregular pieces are formed.
Hardness
Because the chemical composition of garnet varies, the atomic bonds in some species are stronger than in others. As a result, this mineral group shows a range of hardness on the Mohs Scale of about 6.5 to 7.5. The harder species, like almandine, are often used for abrasive purposes.
Pyralspite garnets - Aluminium in Y site
Almandine: Fe3Al2(SiO4)3
Pyrope: Mg3Al2(SiO4)3
Spessartine: Mn3Al2(SiO4)3
Almandine - Almandine, sometimes incorrectly called almandite, is the modern gem known as carbuncle (though originally almost any red gemstone was known by this name). The term "carbuncle" is derived from the Latin meaning "live coal" or burning charcoal. The name Almandine is a corruption of Alabanda, a region in Asia Minor where these stones were cut in ancient times. Chemically, almandine is an iron-aluminium garnet with the formula Fe3Al2(SiO4)3; the deep red transparent stones are often called precious garnet and are used as gemstones (being the most common of the gem garnets). Almandine occurs in metamorphic rocks like mica schists, associated with minerals such as staurolite, kyanite, andalusite, and others. Almandine has nicknames of Oriental garnet, almandine ruby, and carbuncle.
Pyrope - Pyrope (from the Greek pyropós meaning "fire-eyed") is red in color and chemically a magnesium aluminium silicate with the formula Mg3Al2(SiO4)3, though the magnesium can be replaced in part by calcium and ferrous iron. The color of pyrope varies from deep red to almost black. Transparent pyropes are used as gemstones.
A variety of pyrope from Macon County, North Carolina is a violet-red shade and has been called rhodolite, from the Greek meaning "a rose." In chemical composition it may be considered as essentially an isomorphous mixture of pyrope and almandite, in the proportion of two parts pyrope to one part almandite. Pyrope has tradenames some of which are misnomers; Cape ruby, Arizona ruby, California ruby, Rocky Mountain ruby, and Bohemian garnet from the Czech Republic. Another intriguing find is the blue color-changing garnets from Madagascar, a pyrope spessartine mix. The color of these blue garnets is not like sapphire blue in subdued daylight but more reminiscent of the grayish blues and greenish blues sometimes seen in spinel. However, in white LED light the color is equal to the best cornflower blue sapphire, or D block tanzanite; this is due to the blue garnet's ability to absorb the yellow component of the emitted light. Pyrope is an indicator mineral for high-pressure rocks. The garnets from mantle derived rocks, peridotites and eclogites, commonly contain a pyrope variety.
Spessartine - Spessartine or incorrectly spessartite is manganese aluminium garnet, Mn3Al2(SiO4)3. Its name is derived from Spessart in Bavaria. It occurs most often in granite pegmatite and allied rock types and in certain low grade metamorphic phyllites. Spessartine of a beautiful orange-yellow is found in Madagascar (see Mandarin garnet). Violet-red spessartines are found in rhyolites in Colorado and Maine.
Ugrandite group - calcium in X site
Andradite: Ca3Fe2(SiO4)3
Grossular: Ca3Al2(SiO4)3
Uvarovite: Ca3Cr2(SiO4)3
Andradite - Andradite is a calcium-iron garnet, Ca3Fe2(SiO4)3, is of variable composition and may be red, yellow, brown, green or black. The recognized varieties are topazolite (yellow or green), demantoid (green) and melantite (black). Andradite is found both in deep-seated igneous rocks like syenite as well as serpentines, schists, and crystalline limestone. Demantoid has been called the "emerald of the Urals" from its occurrence there, and is one of the most prized of garnet varieties. Topazolite is a golden yellow variety and melanite is a black variety.
Grossular - Grossular is a calcium-aluminium garnet with the formula Ca3Al2(SiO4)3, though the calcium may in part be replaced by ferrous iron and the aluminium by ferric iron. The name grossular is derived from the botanical name for the gooseberry, grossularia, in reference to the green garnet of this composition that is found in Siberia. Other shades include cinnamon brown (cinnamon stone variety), red, and yellow. Because of its inferior hardness to zircon, which the yellow crystals resemble, they have also been called hessonite from the Greek meaning inferior. Grossular is found in contact metamorphosed limestones with vesuvianite, diopside, wollastonite and wernerite. One of the most sought after varieties of gem garnet is the fine green grossular garnet from Kenya and Tanzania called tsavorite. This garnet was discovered in the 1960s in the Tsavo area of Kenya, from which the gem takes its name.
Uvarovite - Uvarovite is a calcium chromium garnet with the formula Ca3Cr2(SiO4)3. This is a rather rare garnet, bright green in color, usually found as small crystals associated with chromite in peridotite, serpentinite, and kimberlites. It is found in crystalline marbles and schists in the Ural mountains of Russia and Outokumpu, Finland.
Less
common species
Calcium in X site
Goldmanite: Ca3V2(SiO4)3
Kimzeyite: Ca3(Zr,Ti)2[(Si,Al,Fe3+)O4]3
Morimotoite: Ca3Ti4+Fe2+(SiO4)3
Schorlomite: Ca3(Ti4+,Fe3+)2[(Si,Ti)O4]3
Hydroxide bearing - calcium in X site
Hydrogrossular: Ca3Al2(SiO4)3-x(OH)4x
Hibschite: Ca3Al2(SiO4)3-x(OH)4x (where x
is between 0.2 and 1.5)
Katoite: Ca3Al2(SiO4)3-x(OH)4x (where x
is greater than 1.5)
Magnesium or manganese in X site
Knorringite: Mg3Cr2(SiO4)3
Majorite: Mg3(Fe,Al,Si)2(SiO4)3
Calderite: Mn3Fe3+2(SiO4)3
Knorringite - Knorringite is a magnesium chromium garnet species with the formula Mg3Cr2(SiO4)3. Pure endmember knorringite never occurs in nature. Knorringite is only formed under high pressure and is often found in kimberlites. It is used as an indicator mineral in the search for diamonds.
Synthetic garnets
In yttrium iron garnet (YIG), Y3Fe2(FeO4)3, the five iron(III) ions occupy two octahedral and three tetrahedral sites, with the yttrium(III) ions coordinated by eight oxygen ions in an irregular cube. The iron ions in the two coordination sites exhibit different spins, resulting in magnetic behaviour. YIG is a ferrimagnetic material having a Curie temperature of 550 K. By substituting specific sites with rare earth elements, for example, interesting magnetic properties can be obtained. One example for this is gadolinium gallium garnet, Gd3Ga2(GaO4)3, which is synthesized for use in magnetic bubble memory. Yttrium aluminium garnet (YAG), Y3Al2(AlO4)3, is used for synthetic gemstone. When doped with neodymium (Nd3+), these YAl-garnets are useful as the lasing medium in lasers.
Geological importance of garnet
The Garnet group is a key mineral in interpreting the genesis of many igneous and metamorphic rocks via geothermobarometry. Diffusion of elements is relatively slow in garnet compared to rates in many other minerals, and garnets are also relatively resistant to alteration. Hence, individual garnets commonly preserve compositional zonations that are used to interpret the temperature-time histories of the rocks in which they grew. Garnet grains that lack compositional zonation commonly are interpreted as having been homogenized by diffusion, and the inferred homogenization also has implications for the temperature-time history of the host rock.
Garnets are also useful in defining metamorphic facies of rocks. For instance, eclogite can be defined as a rock of basalt composition, but mainly consisting of garnet and omphacite. Pyrope-rich garnet is restricted to relatively high-pressure metamorphic rocks, such as those in the lower crust and in the Earth's mantle. Peridotite may contain plagioclase, or aluminium-rich spinel, or pyrope-rich garnet, and the presence of each of the three minerals defines a pressure-temperature range in which the mineral could equilibrate with olivine plus pyroxene: the three are listed in order of increasing pressure for stability of the peridotite mineral assemblage. Hence, garnet peridotite must have been formed at great depth in the earth. Xenoliths of garnet peridotite have been carried up from depths of 100 km and greater by kimberlite, and garnets from such disaggegated xenoliths are used as a kimberlite indicator minerals in diamond prospecting. At depths of about 300 to 400 km and greater, a pyroxene component is dissolved in garnet, by the substitution of (Mg,Fe) plus Si for 2Al in the octahedral (Y) site in the garnet structure, creating unusually silica-rich garnets that have solid solution towards majorite. Such silica-rich garnets have been identified as inclusions within diamonds.
Uses of garnets
Pure crystals of garnet are used as gemstones. Garnet sand is a good abrasive, and a common replacement for silica sand in sand blasting. Mixed with very high pressure water, garnet is used to cut steel and other materials in water jets. Garnet sand is also used for water filtration media.