Lessico
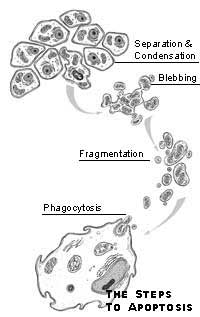
Apoptosi
Morte cellulare programmata
Nel 1972 Kerr, Wyllie & Currie proposero di attribuire il termine di apoptosi anche all'evento biologico che va sotto il nome di morte cellulare programmata e che gioca un ruolo complementare, ma opposto, a quello della mitosi nel regolare la popolazione delle cellule di un organismo, entrando in gioco, per esempio, anche quando è necessario regolare l'estensione di una membrana interdigitale.
Il termine apoptosi fu in precedenza usato per etichettare la caduta dei petali dai fiori oppure delle foglie dalle piante, in quanto derivato dal greco ἀπόπτωσις che significa caduta, distacco, a sua volta derivato dal verbo ἀποπίπτω che equivale a cadere, saltare giù.
L'articolo di George Johnson apparso nel 1996 sul New York Times dimostra che sperimentalmente i polli possono venir dotati di zampe da palmipede, e pertanto nulla esclude che ciò possa verificarsi in modo naturale grazie a qualche balzana mutazione genetica. La Natura è maestra in queste cose.
Come
affermano Abarca-Buis, Rios-Flores y Chimal-Monroy, autori messicani di Control molecular de la apoptosis durante la morfogénesis
de la extremidad (apparso in Mensaje Bioquímico, Vol. XXIX - 2005), il pollo si è prestato in modo
eccellente per lo studio dell'apoptosi, un processo biologico che rende
ragione non solo del fenotipo di mutazioni letali come il talpid3![]() o mutazione talpa, ma anche per quello della mancanza
di ali
o mutazione talpa, ma anche per quello della mancanza
di ali![]() – wingless, wg – e per
i vari fenotipi della polidattilia
– wingless, wg – e per
i vari fenotipi della polidattilia![]() .
Di questo esauriente lavoro messicano riportiamo la traduzione solo della
parte che ci riguarda,
cioè quella relativa al pollo. Per i dati ultraspecialistici riguardanti i
meccanismi biochimici implicati nell'apoptosi, si mette a disposizione
l'articolo completo in castigliano in formato PDF
.
Di questo esauriente lavoro messicano riportiamo la traduzione solo della
parte che ci riguarda,
cioè quella relativa al pollo. Per i dati ultraspecialistici riguardanti i
meccanismi biochimici implicati nell'apoptosi, si mette a disposizione
l'articolo completo in castigliano in formato PDF![]() .
.
Anche in campo umano entra fisiologicamente in ballo l'apoptosi, ma questo meccanismo può alterarsi e potrebbe giustificare, a mio avviso, la comparsa di mani e piedi anomali in corso di patologie di origine genetica: la sindrome di Timothy e la sindrome di Apert.
Se abbiamo un po' di tempo da spendere per aggiornarci in biologia, vale la pena leggere quanto riportato dall'enciclopedia Encarta e poi due belle monografie: una dovuta a due studiosi canadesi e l'altra ad alcuni medici della Columbia University di New York.
Apoptosi o Morte cellulare
Fenomeno fisiologico che comporta la degenerazione della cellula a conclusione del suo ciclo vitale; è di grande interesse per le sue implicazioni nei processi di invecchiamento e nello sviluppo di tumori o malattie degenerative. Alcuni autori definiscono il fenomeno con le espressioni “morte programmata” o “suicidio cellulare”; in effetti, ha senso usare i termini “programmata” e “suicidio” perché, come si è osservato, nel patrimonio genetico della cellula entrano in gioco alcuni geni specifici, prima inattivi, che portano la cellula ad autodistruggersi.
Cenni storici
Il termine apoptosi fu utilizzato per la prima volta nel 1972 dal patologo australiano John Foxton Ross Kerr e dal biologo Jeffrey Searle, suo connazionale. Nel 1965 Kerr aveva iniziato le sue ricerche sulle cellule di fegato di ratto, nelle quali osservò una forma inconsueta di degenerazione o necrosi. Nei due decenni seguenti, identificata l’apoptosi come fenomeno differente dalla necrosi, gli studi di Kerr e di molti altri autori si moltiplicarono allo scopo di chiarire le relazioni tra l’apoptosi e i processi di sviluppo embrionale, spermatogenesi, cancerogenesi e la capacità di autoriparazione dei tessuti. In seguito, l’apoptosi divenne materia di ricerche anche nel campo dell’immunologia e della biochimica; furono riconosciute intere famiglie di enzimi coinvolte nella morte cellulare e fattori di crescita capaci di stimolare la cellula e impedire l’attivazione dei meccanismi di autodistruzione.
Finora
sono stati descritti gran parte dei meccanismi con cui la cellula “esegue”
la propria morte, mentre restano ancora da chiarire molti dei processi
genetici e biochimici di regolazione e induzione, che operano a monte e
“comandano” l’apoptosi di una determinata cellula. Di grande rilevanza
le recenti scoperte effettuate dal biologo statunitense H. Robert Horvitz, dal
biologo molecolare Sidney Brenner e dal biologo John E. Sulston, britannici,
sul nematode Caenorabditis elegans![]() ,
per le quali i tre scienziati furono insigniti nel 2002 del premio Nobel. Allo
stesso Kerr fu attribuito, nel 2000, il Paul Ehrlich and Ludwig Darmstaedter
Prize (condiviso con Horvitz), per aver identificato il fenomeno dell’apoptosi,
onorificenza considerata seconda solo al Nobel.
,
per le quali i tre scienziati furono insigniti nel 2002 del premio Nobel. Allo
stesso Kerr fu attribuito, nel 2000, il Paul Ehrlich and Ludwig Darmstaedter
Prize (condiviso con Horvitz), per aver identificato il fenomeno dell’apoptosi,
onorificenza considerata seconda solo al Nobel.
La morte cellulare è attualmente ritenuta uno dei fronti più interessanti della ricerca biologica e genetica; dalla comprensione dei suoi meccanismi si auspica possano derivare applicazioni nella chemioterapia, nel trattamento del cancro, delle malattie autoimmuni e neurodegenerative.
Apoptosi e Necrosi
L’apoptosi non deve essere confusa con la necrosi, processo anch’esso responsabile della morte delle cellule, senza che ciò comporti necessariamente la morte dell’organismo (si pensi, ad esempio, alla necrosi di alcuni tessuti a causa di congelamento o infarto). Nella necrosi si osserva la lisi (cioè la disgregazione) della cellula: il nucleo degenera e la membrana plasmatica si rompe riversando all’esterno il contenuto; ciò determina una reazione immunitaria dell’organismo e una risposta infiammatoria. La necrosi è dunque un fenomeno patologico. Nell’apoptosi, invece, il nucleo degenera ma la membrana non si rompe; la cellula viene fagocitata dalle cellule specializzate (fagociti). La cellula, insomma, originatasi dalla divisione di una cellula-madre, si differenzia in cellula adulta e va incontro a un certo numero di divisioni mitotiche, secondo il tipo cui essa appartiene; quindi, subisce una fase di quiescenza e senescenza e, infine, l’apoptosi.
Funzioni dell'apoptosi
La morte programmata delle cellule è fondamentale nel controllo numerico delle cellule stesse; le cellule in apoptosi, infatti, bilanciano quelle che si dividono per mitosi. Tale equilibrio contribuisce all’omeostasi di ciascun tessuto dell’organismo e assicura il normale ricambio delle cellule, soprattutto nei tessuti epiteliali soggetti a rapido rinnovamento. Quando l’equilibrio viene alterato da un fenomeno patologico, se prevale la componente mitotica si originano fenomeni di ipertrofia, cioè di aumento abnorme del numero di cellule, come nei tumori; se invece prevale l’apoptosi il tessuto tende ad atrofizzarsi.
L’alterazione dell’equilibrio tra mitosi e apoptosi può avvenire anche fisiologicamente, ad esempio quando l’organismo deve promuovere lo sviluppo o l’eliminazione di una determinata struttura. Un caso importante è quello dello sviluppo embrionale, in cui dopo un periodo iniziale di intensissima attività mitotica, l’apoptosi interviene nel riassorbimento di strutture fetali come, ad esempio, le membrane tra le dita o le cellule nervose tra le quali non si siano create connessioni sinaptiche. Le cellule entrano in apoptosi anche per circoscrivere gli eventuali danni causati da una sostanza, ad esempio un composto tossico, radiazioni ionizzanti, farmaci, radicali liberi, capaci di indurre mutazione, oppure se vengono infettate da virus: infatti, se i danni, soprattutto a carico del DNA, non possono essere riparati, le cellule potrebbero non svolgere più la loro funzione all’interno del tessuto e danneggiarlo, oppure trasformarsi in cellule neoplastiche (tumorali) o diventare veicolo di propagazione del virus. Dunque, l’apoptosi assume in questo caso una funzione protettiva dell’intero organismo. Da notare che alcuni virus, a loro volta, sono in grado di produrre fattori inibitori dell’apoptosi, affinché la cellula continui a sopravvivere (e a “lavorare” per loro): si tratta di un interessante caso di coevoluzione.
Microsoft
® Encarta ® 2006. ©
1993-2005 Microsoft Corporation.
Tutti i diritti riservati.
The
Chicken With a Duck's Feet:
It's All in the Biochemical Signal
By
George Johnson
Science
Desk - New York Times
Published: May 21, 1996
|
Mentre
gli scienziati rimettono ordine nel complesso origami biochimico
attraverso il quale un foglio in bianco di cellule indifferenziate
viene rinchiuso all'interno di una creatura completamente sviluppata,
essi stanno imparando una sconcertante lezione: il contesto è tutto.
Ciò che rappresenta una deformità in un organismo, per un altro può
rappresentare un dono dell'evoluzione. Si
prenda il caso della sindattilia, cioè
la presenza di membrana interdigitale alla mano e al piede. Ciò
che in una persona viene considerata una lieve deformità, nell'anatra
espande il suo territorio tanto da farle abbracciare sia l'acqua che
il cielo. Come
mai le estremità degli arti di alcune creature sfociano in mani o
piedi con dita ben definite, mentre altre si traducono in pagaie? In
un rapporto pubblicato sul numero del 3 maggio della rivista Science,
alcuni ricercatori del Memorial
Sloan-Kettering Cancer Center
di Manhattan hanno detto di aver trovato una buona parte della
risposta: si tratta di un segnale biochimico che pare controlli il
manifestarsi o meno della membrana interdigitale. In
un lampante esperimento il Dr. Hongyan Zou e la Dsa Lee Niswander
hanno bloccato tale segnale e hanno trovato che così i polli
sviluppano piedi di anatra. "L'effetto è veramente molto
drammatico", ha detto la Dsa Niswander in un'intervista. Nel suo
lavoro ha messo in evidenza che bloccando il segnale molecolare
rappresentato dalle proteine morfogenetiche dell'osso – BMP, bone
morphogenetic proteins – ha prodotto la deformità in
"praticamente il 100%" degli arti sui quali stava conducendo
l'esperimento. Altri
laboratori hanno trovato che le BMP sono importanti in altri distretti
di un organismo in via di sviluppo, in quanto dicono a un gruppo di
cellule se formare un pollice o un mignolo, e sono pure importanti
nell'orchestrare lo sviluppo di reni, occhi e altri tessuti. In
passato erano interessate a queste molecole soprattutto le società di
biotecnologie nella speranza di guadagni con farmaci che stimolano la
crescita dell'osso, ma queste proteine stanno dimostrando di essere
così ubiquitarie durante lo sviluppo embrionale che alcuni
ricercatori desiderano poter dar loro un nome più eloquente. "È
veramente un gruppo affascinante di molecole" ha detto la Dsa
Brigid Hogan, biologa dello sviluppo presso l'Howard
Hughes Medical Institute della
Vanderbilt University. "Probabilmente sono importanti per
lo sviluppo di qualunque organo." L'anno
scorso il laboratorio della Dsa Hogan ha scoperto che se in un
embrione in via di sviluppo il gene che codifica per un certo tipo di
molecola di BMP viene cancellato, l'organismo morirà senza formare lo
strato medio delle cellule, cioè il mesoderma, che dà origine a una
varietà di tessuti come muscolo, rene, cartilagine e osso. Più
recentemente, in un'indagine in corso non ancora pubblicata, il suo
laboratorio ha scoperto che mettendo fuori combattimento il gene per
un altro tipo di BMP si impedisce ai topi di produrre sperma. Un'altra
ricercatrice, la Dsa Elizabeth Robertson, biologa all'Università di
Harvard, ha trovato che mettendo fuori combattimento i geni per le BMP
si sono prodotte delle deformità dell'occhio e del rene nonché delle
malformazioni ai piedi. "La
domanda è: come fa un tipo di molecola giocare tutti questi
differenti ruoli?" ha detto la Dsa Cheryll Tickle, una studiosa
di biologia dello sviluppo presso l'University
College di Londra. Ha detto che lo studio della Dsa.
Niswander sarebbe importante nel districare i vari percorsi chimici
coi quali le BMP pilotano la trasformazione di un uovo in un neonato.
"Potrebbe trattarsi di un equilibrio molto complicato" ha
detto. In alcuni stadi di sviluppo le BMP sono implicate nella morte
cellulare programmata, cioè la rimozione di tessuto indesiderato. Ma
esse hanno pure altri ruoli. Come
se i congegni della natura non fossero già abbastanza complessi,
alcuni ricercatori hanno trovato che altri geni sono pure implicati
nello sviluppo di un arto. Nel numero del 26 aprile di Science
i Dottori. Yasuteru Muragaki, Stefan Mundlos, Joseph Upton e Bjorn R.
Olsen della Harvard Medical School hanno descritto come avevano ricollegato una deformità umana
chiamata sinpolidattilia – membrana interdigitale accompagnata da
dita soprannumerarie - a una mutazione del gene detto homeobox.
Si è visto per la prima volta che geni homeobox controllano lo
sviluppo del moscerino della frutta, la Drosophila melanogaster:
una mutazione in uno di tali geni può far sì che le zampe spuntino
sulla testa là dove dovrebbero esserci le antenne. A partire dalla
scoperta dei geni homeobox, geni corrispondenti sono stati
trovati in tutto il regno animale. Il
Dr. Mundlos ha detto che il suo gruppo di ricercatori potrebbe aiutare
nel rispondere ad alcune intricate domande sui modi molto diversi con
cui gli arti possono svilupparsi. "Perché il pesce ha le pinne,
mentre il pollo ha dei piedi
con tre dita davanti e uno dietro, mentre noi abbiamo cinque
dita?" ha chiesto. "Noi crediamo che la mutazione che
abbiamo trovato getti luce su questo quesito." I
geni homeobox producono molecole denominate fattori di
trascrizione, che attivano e inattivano altri geni. Le dita
addizionali della mano e del piede della sinpolidattilia sembrano
scaturire quando una mutazione fa sì che il gene homeobox
inserisca parecchie copie di un aminoacido, l'alanina, in una regione
del fattore di trascrizione che sembra essere implicato con le dita in
accrescimento. I topi - e gli esseri umani con le loro mani a cinque
dita – hanno una sequenza di 15 alanine nella proteina dell'homeobox.
I polli ne hanno 9, mentre il pesce zebra non ne ha neppure una. Il
Dr Mundlos ha detto che non era ancora chiaro come i risultati
ottenuti dal suo gruppo sullo sviluppo di un arto fossero in relazione
con le recenti scoperte sulle BMP. "Tutte queste molecole sono in
stretta relazione una con l'altra", ha detto. "Abbiamo
un'evidenza piuttosto buona che tutte quante hanno un qualcosa a che
fare con un modello molto precoce." Mentre
i geni homeobox producono fattori di trascrizione che operano
all'interno del nucleo della cellula, le molecole BMP vengono usate
per inviare segnali da cellula a cellula. Un segnale homeobox
potrebbe dire a un gene di fabbricare una BMP. Oppure, di converso,
una BMP potrebbe far sì che una cellula dia delle istruzioni al suo
nucleo per attivare un gene homeobox. Il tutto fa parte della
complessa orchestrazione dello sviluppo. Il
trucco della Dsa Niswander di trasformare piedi di pollo in piedi di
anatra fu inspirato dalla ricerca degli ultimi cinque anni, la quale
indica che i geni sono impegnati nel costruire molecole segnale di BMP
nel tessuto interdigitale di mani e piedi. Gli scienziati di molti
laboratori hanno stabilito ciò attraverso una tecnica chiamata
ibridazione dell'RNA in situ. Dopo che un gene è attivato, le sue
istruzioni genetiche vengono copiate in molecole di RNA messaggero che
mandano le informazioni ai ribosomi extranucleari dove vengono
sintetizzate le proteine. Per determinare se un gene è attivo, si
deve semplicemente cercare il suo RNA messaggero. Per
effettuare la ricerca di geni attivi per le BMP, gli sperimentatori
hanno confezionato degli agglomerati
di RNA in modo che le loro sporgenze e gli incavi chimici
formassero un'immagine speculare delle corrette molecole di RNA
messaggero per le BMP. All'RNA fabbricato fu quindi assegnata
un'etichetta chimica in modo da poter essere facilmente rintracciato e
identificato. Quando gli scienziati immersero un embrione di pulcino o
di topo nell'RNA fabbricato, esso ha messo in luce e ha chiarito i
suoi ruoli complementari, o immagini speculari. Allora i ricercatori
impiegarono degli anticorpi contro l'etichetta chimica per concentrare
l'attenzione proprio dove a livello somatico le molecole di RNA
stavano congregandosi. Questi erano i punti dove il gene della BMP era
stato attivato. Gli scienziati sospettarono che nella maggior parte
degli animali le molecole di BMP impedissero il formarsi delle
membrane interdigitali scatenando la morte cellulare programmata.
Quando ricevono un segnale dalle BMP, le cellule interdigitali
compiono un suicidio di massa, permettendo lo sviluppo di dita
indipendenti. Nelle anatre -
e a quanto pare, anche nelle persone con sindattilia - ciò non
si verifica. La
Dsa Niswander decise di vedere cosa accadrebbe se avesse bloccato il
segnale del suicidio cellulare. Molecole come le BMP liberano il loro
messaggio legandosi a una molecola recettrice che si fissa all'esterno
della membrana esterna di una cellula. Quando la molecola di BMP si
lega al recettore, ha inizio una cascata di reazioni chimiche interne
che in ultima analisi dice al nucleo di creare gli enzimi necessari
per eseguire una mansione specifica. Per
eliminare il segnale BMP, la Dsa Niswander e i suoi colleghi usarono
un tipo di terapia genica inversa. Dapprima provocarono una mutazione
nel gene che fabbrica i recettori BMP. Inserirono il gene mutato in un
retrovirus che inserì il gene alterato nelle cellule di embrioni di
pulcino. Gli embrioni col gene mutato produssero dei recettori BMP
defettivi che erano sordi al segnale del suicidio. Come
era prevedibile, i pulcini svilupparono tra le dita del piede delle
membrane interdigitali simili a quelle delle anatre. Quindi,
usando la tecnica di ibridazione per scoprire dove i geni BMP erano
attivi, gli scienziati rivolsero la loro attenzione alle anatre e
trovarono che mentre i geni si erano attivati in altre parti del
corpo, non si erano attivati nei
tessuti interdigitali. Gli
scienziati trovarono pure che la mancanza di BMP portò nei pulcini a
un altro cambiamento: essi tendevano a sviluppare delle penne ai piedi
invece di squame. Gli scienziati sono dell'avviso che durante lo
sviluppo certe cellule sono in grado di assumere identità differenti.
Se abbandonate a se stesse, diventano penne. Se stimolate
delicatamente dalle BMP diventano squame. Gli
scienziati ritengono che il sistema di segnalazione scoperto nei
pulcini è attivo anche negli esseri umani. Quando la natura va a
inciampare in un trucco utile, tende a conservarlo. "Sia il
segnale cellulare che molecolare sembrano essere molto ben conservati
nel pulcino e nel topo", ha detto la Dsa Niswander.
"Presumibilmente questo è vero
anche negli esseri umani." I
dettagli su come veramente la morte cellulare programmata sia in grado
di plasmare gli arti rimane un mistero, ha detto il Dr Matthew P.
Scott, professore di biologia dello sviluppo e genetica all'Università
di Stanford e ricercatore dell'Howard
Hughes Medical
Institute. "Adesso abbiamo bisogno di sapere come i
segnali BMP si producono nelle giuste aree", ha detto. "Ma
non potremmo porci questa domanda senza il lavoro di Zou e Niswander". Le
implicazioni dell'esperimento possono andare al di là del campo
dell'embriologia. La morte cellulare programmata è importante anche
nel modellare altre parti del corpo, incluso il cervello, dove nel
feto e nel neonato una profusione di neuroni è spazzata via per
lasciar posto al circuito dei neuroni. Si è ipotizzato che anche la
BMP è coinvolta in tale processo. Ma
le molecole giocano anche dei ruoli che sembrano non aver nulla a che
fare col suicidio cellulare. "La cosa interessante a proposito
delle BMP è che possono svolgere azioni diverse in
stadi diversi di sviluppo embrionale" ha detto il Dr.
Hogan Vanderbilt. "In alcuni contesti promuovono la morte
cellulare, in altri la sopravvivenza, a seconda di dove e quando
vengono espresse." |
|
Pit-1 homeobox-containing protein bound to DNA A
homeobox (or homoeobox) is a DNA sequence found within genes that are
involved in the regulation of development (morphogenesis) of animals,
fungi and plants. Genes that have a homeobox are called homeobox genes
and form the homeobox gene family. A
homeobox is about 180 base pairs long; it encodes a protein domain
(the homeodomain) which can bind DNA. Homeobox genes encode
transcription factors which typically switch on cascades of other
genes, for instance all the ones needed to make a leg. The homeodomain
binds DNA in a specific manner. However, the specificity of a single
homeodomain protein is usually not enough to recognize only its
desired target genes. Most of the time, homeodomain proteins act in
the promoter region of their target genes as complexes with other
transcription factors, often also homeodomain proteins. Such complexes
have a much higher target specificity than a single homeodomain
protein. A
particular subgroup of homeobox genes are the Hox genes, which are
found in a special gene cluster, the Hox cluster (also called Hox
complex). Hox genes function in patterning the body axis. Thus, by
providing the identity of particular body regions, Hox genes determine
where limbs and other body segments will grow in a developing fetus or
larva. Mutations in any one of these genes can lead to the growth of
extra, typically non-functional body parts in invertebrates, for
example aristapedia complex in Drosophila, which results in a
leg growing from the head in place of an antenna and is due to a
defect in a single gene (this mutation is also known as Antennapedia).
Mutation in vertebrate Hox genes usually results in spontaneous
abortion. The
homeobox genes were first found in the fruit fly Drosophila
melanogaster and have subsequently been identified in many other
species, from insects to reptiles and mammals. The diagram to the
right is a structural model of the Rattus norvegicus Pit-1
homeobox-containing protein (purple) bound to DNA. Pit-1 is a
regulator of growth hormone gene transcription. Pit-1 is a member of
the POU DNA-binding domain family of transcription factors so it can
bind to DNA using both the POU domain and the homeodomain. Homeobox
genes have even been found in fungi, for example the one-cellular
yeasts, and plants. The well known homeotic genes in plants (MADS-box
genes) are not homologous to Hox genes in animals. Plants and animals
do not share the same homeotic genes, and this suggests that homeotic
genes were evolved once in the early evolution of animals and once
again in the early evolution of plants. Humans generally contain
homeobox genes in four clusters, called HOXA (or sometimes HOX1), HOXB,
HOXC, or HOXD, on chromosomes 7, 17, 12, and 2, respectively. Mutations
to homeobox genes can produce easily visible phenotypic changes. Two
examples of homeobox mutations in the above-mentioned fruit fly are
legs where the antennae should be, and a second pair of wings.
Duplication of homeobox genes can produce new body segments, and such
duplications are likely to have been important in the evolution of
segmented animals. Interestingly, there is one insect family, the
xyelid sawflies, in which both the antennae and mouthparts are
remarkably leg-like in structure. |
Control
molecular de la apoptosis
durante la morfogénesis de la extremidad
Mensaje
Bioquímico,
Vol. XXIX (2005)
René
Fernando Abarca-Buis, Alberto Jesús
Rios-Flores, Jesús
Chimal-Monroy
Instituto
de Investigaciones Biomédicas, Universidad Nacional autónoma
de México
Apartado
Postal 70-228 México DF 04510
Introduzione
Durante lo sviluppo degli organismi pluricellulari si generano distinti tipi cellulari che daranno origine a differenti tessuti e organi. Così il bilancio corretto tra differenziazione, proliferazione e morte cellulare determinano il destino delle varie linee cellulari e il numero di cellule che danno forma a un organo. L'apoptosi o morte cellulare programmata è parte essenziale della vita dei metazoi: spesso negli organismi si produce un numero maggiore di cellule rispetto a quelle necessarie e allora l'apoptosi svolge un ruolo importante eliminando cellule che si trovano in eccesso o per dare un abbozzo ai tessuti.
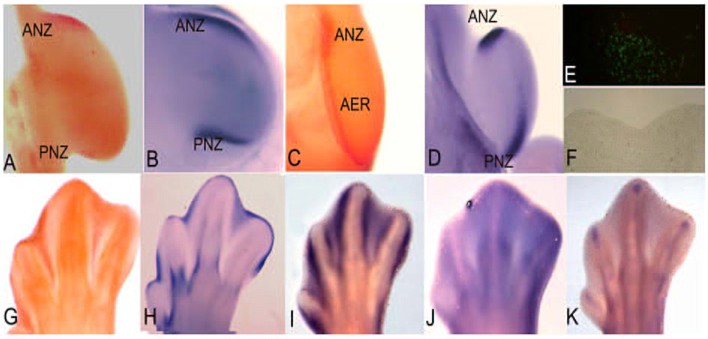
figura 1
L'estremità
embrionale del pollo
un modello per lo studio dell'apoptosi
L'estremità del pollo in via di sviluppo si è dimostrata essere uno dei modelli più ricorrenti per lo studio dell'apoptosi in quanto presenta diverse zone che vengono eliminate attraverso questo processo.
La zona necrotica anteriore (ANZ – Anterior Necrotic Zone) e la zona necrotica posteriore (PNZ – Posterior Necrotic Zone) sono aree del mesoderma che, rispettivamente, circondano il bordo anteriore e posteriore delle estremità embrionali (fig. 1 A).
Nonostante il termine con cui sono denominate, queste zone vengono eliminate per apoptosi già in tappe precoci e sono state messe in relazione con la fisiologica riduzione del numero delle dita negli uccelli, tre dita nelle ali e quattro nelle zampe.
In alcune mutazioni del pollo che presentano polidattilia, cioè un incremento del numero delle dita, non si osservano queste zone apoptosiche [1]. Inoltre, il pollo mutante caratterizzato dall'assenza di ali, wingless, presenta durante il suo sviluppo embrionale una ANZ abbastanza estesa correlata appunto con la perdita delle ali [2].
Occorre segnalare che nei mammiferi con pentadattilia, cioè dotati di cinque dita, non compaiono queste aree di apoptosi.
Il buco nero è rappresentato da un'altra zona presente nel mesenchima centrale dell'estremità embrionale di uccelli e mammiferi che si pensa essere in relazione con la separazione dell'ulna e del radio per le estremità anteriori, della tibia e della fibula per quelle posteriori. Il pollo mutante talpid3 non ha quest'area di apoptosi e mostra la fusione di queste due ossa [3].
Per le dimensioni e la loro accessibilità, le aree apoptosiche delle zone interdigitali del pollo rappresentano il modello più usato per studiare i meccanismi dell'apoptosi in vivo.
Come indica il nome, le zone interdigitali sono localizzate fra le dita (fig. 1 E-G) e, a seconda della specie, queste zone si eliminano oppure permangono durante la vita adulta dell'organismo, partecipando in maniera importante alla morfogenesi delle estremità degli adulti. Per esempio, in specie con dita separate come il pollo [4], la lucertola [5], il topo e l'uomo [6, 7] l'apoptosi si estende in tutto lo spazio interdigitale, ma nell'anatra o nella tartaruga che presentano questo tessuto in modo permanente, l'apoptosi si attiva solo nella parte più distale [8, 5].
Oltre che nel mesoderma, l'apoptosi è attivata anche nell'ectoderma, in una regione molto importante per lo sviluppo dell'estremità, denominata Cresta Ectodermica Apicale (AER Apical Ectodermic Ridge). (Fig 1 C). L'AER è un ingrossamento dell'ectoderma localizzato nella parte più distale dell'estremità embrionale ed è una struttura molto importante poiché governa la crescita prossimo-distale dell'estremità. L'eliminazione dell'AER provoca il troncamento dell'estremità, mentre la presenza di due AER dove originariamente ce n'è una, ne provoca la biforcazione [9]. Si è osservato che l'apoptosi regola il volume dell'AER in modo tale da poter realizzare la sua funzione in modo molto preciso durante tutto lo sviluppo dell'estremità. In base a questo, l'incremento di cellule apoptosiche nell'AER risulta in un troncamento parziale o completo dell'estremità [10], processo che nei serpenti si presenta in modo naturale [11]. In base a queste osservazioni, si è dedotto che in tappe dello sviluppo durante le quali ha luogo la formazione delle dita, si manifesta una polidattilia oppure una riduzione del numero delle dita in base a un'inibizione o a un incremento dell'apoptosi nell'AER di embrioni di topo [12]. Tutte queste osservazioni mettono in luce l'importante ruolo dell'apoptosi affinché le estremità si formino in modo corretto. In base a ciò, nella figura 1 mostriamo l'espressione di alcuni geni la cui espressione è in relazione con le zone di apoptosi a livello delle estremità e la cui funzione è in relazione diretta con il controllo di questo processo.
1. Hinchliffe, J.R. y Ede, D.A. (1967).
Limb development in the polydactylous talpid3 mutant of the fowl. J.
Embryol. Exp. Morphol.17: 385-404.
2. Hinchliffe, J.R y Ede, D.A. (1973). Cell death and the
development of limb form and skeletal pattern in normal and wingless (ws)
chick embryos. J. Embryol. Exp. Morphol. 30: 753-772.
3. Hinchiliffe, J R. y Thorogood, P V. (1974). Genetic
inhibition of mesenchymal death and the development of form and skeletal
pattern in the limbs of talpid3 (ta3) mutante chick embryos. J. Embryol. Exp.
Morphol. 31: 747-760.
4. Pautou, M. P. (1975). Morphogenesis of the chick embryo
foot. J. Embryol. Exp. Morphol. 34: 511- 529.
5. Fallon, J.F. y Cameron, J. (1977). Interdigital cell death
during limb development of turtle and lizard with an interpretation of
evolutionary significance. J. Embryol. Exp. Morphol. 40: 285-289.
6. Milaire, J. (1977). Histochemical expression of
morphogenetic gradients during limb morphogenesis. Birth defects: original
article series. 13: 37-67.
7. Kelley, R.O. (1970). An electron microscopic study of
mesenchyme during development of interdigital spaces in man. Anat. Rec. 168:
43-53.
8. Saunders, J.W. y Fallon, J.F. Cell death in morphogenesis.
In: Locke M, editor. Major problems in developmental biology. New York:
Academic Press; 1967. p. 289-314.
9. Saunders Jr. J.W. (1972). Developmental control of
three-dimensional polarity in the avian limb. Ann. NY Acad. Sci. 193: 29-42.
10. Seto, M.L., Nunes, M.E., MacArthur, C.A., Cunningham,
M.L. (1997) Pathogenesis of ectrodactyly in the dactylaplasia mouse: aberrant
cell death of the apical ectodermal ridge. Teratology. 56: 262-270.
11. Raynaud A. Developmental mechanism involved in the
embryonic reduction of limbs in reptiles. (1990) Int. J. Dev Biol. 34:
233-243.
12. Klein, K.L., Scott, W.J., Wilson, J.G. (1981).
Aspirin-induced teratogenesis: a unique pattern of cell death and subsequent
polydactyly in the rat. J. Exp. Zool. 216: 107-112.
Monografia canadese sull'apoptosi
Essendo scritto in un inglese assai facile, ecco l'esauriente e ottima monografia sull'apoptosi desunta dal web e stilata da Michael Wride & Leon Browder dell'Università di Calgary, provincia dell'Alberta, Canada sudoccidentale.
|
Patologie
genetiche umane Sindrome
di Timothy
|
Death
of a Cell by Suicide
In
the life sciences, death has become a hot topic
By
William Allstetter Columbia
University Medical Center
Most
people have seen a cell divide. Short movies showing the sequential
phases of mitosis are a mainstay of science education. But few have
seen a cell die. That may be because, until recently, few people paid
much attention to cell death. Most
scientists considered it the final, relatively dull endgame of a cell's
existence. They believed that once toxic insult or injury destroys a
cell's ability to carry on metabolism and maintain homeostasis, there
is nothing to be done. The cell and its organelles swell, their
membranes break apart, and the cell's contents spill into the
intercellular space. Immune system cells and molecules sweep in to
clean up the mess, causing inflammation and additional damage in the
process. Necrosis, as it is called, was described long ago and offered
little of interest to researchers. In
the 1990s, however, it has become abundantly clear that cell death is
considerably more complex and interesting. Cells don. t always wait
for deadly forces to do them in. They commit suicide, or, in a more
scientific term, apoptosis. In
fact, each cell in our bodies contains a complex machinery of death. A
host of genes, receptors, and enzymes carefully controls when and
under what conditions a cell will kill itself. Some cells initiate
apoptosis after chemical messengers bind to their "death
receptors." In others, suicide is the default, preventable only
by a constant stream of reassuring messages from other cells. Cells
also respond to internal signals, condemning to death any cell that
poses a threat to the larger organism. "Apoptosis
is a normal developmental and safety process," says Dr. Michael
Shelanski, Delafield Professor and Chairman of Pathology. "It
gets sick cells out of the way and makes sure we develop properly." The
hand of a developing fetus begins as a flat paddle. Apoptosis sculpts
it into individual fingers. A developing brain makes more than twice
the neurons it will eventually employ; ones that fail to make the
right connections are eliminated. When genetic damage occurs, internal
sentries, such as p53, halt cell division until repairs can be made.
If the damage is beyond repair, suicide is invoked. Cells in the gut,
skin, and elsewhere commit suicide every day as part of the normal
maintenance of tissue. But
when the death machinery goes awry, disease can result. In
neurodegenerative disorders, autoimmune disorders, and stroke, cells
die prematurely. In cancer, cells fail to die when they should. Today,
apoptosis is one of the hottest topics in biomedical research. Basic
scientists have a whole new phenomenon to examine and describe.
Clinical scientists are hoping to cure several diseases by preventing
or inducing apoptosis. Thousands of papers on apoptosis are published
every year, and entire conferences are organized around individual
elements of the process. "Something
about it intuitively appeals," says Dr. Beth Levine, assistant
professor of medicine. "It has implications for so many aspects
of biology." Some
complain that apoptosis has become the latest research bandwagon, with
scientists seeing it where it doesn't exist and scrambling to include
it in their grant applications. A handful of P&S researchers,
however, were ahead of the curve, investigating apoptosis before it
became the latest biological buzzword. They helped reveal basic
elements of apoptosis in all cells. They have discovered how and where
it occurs in both healthy and diseased tissue. They have even
demonstrated its role in interspecies competition. Several are now
developing therapies based on apoptosis. Apoptosis
was first described in a 1972 paper by John Kerr, Andrew Wyllie, and
Alastair Currie, three researchers at the University of Aberdeen. The
researchers also coined the term, which comes from the Greek word that
describes the falling of leaves from a tree or petals from a flower.
In contrast to the messy process of necrosis, apoptosis is quick and
neat. Instead of swelling, a cell undergoing apoptosis shrivels and
separates from its neighbors. Its DNA and organelles condense. The
cell then "blebs," dividing into several small vesicles,
which are consumed by neighboring cells. Apoptosis can occur in as
little as 20 minutes and leaves no trace behind, which may explain why
biologists failed for so long to see it. Although
the paper by Kerr, Wyllie, and Currie is now considered a classic,
their concept of apoptosis languished, largely unnoticed, for many
years. Then, in the mid-1980s Robert Horvitz at the Massachusetts
Institute of Technology began investigating cell death in the nematode
worm, C. elegans. He learned that precisely 131 of the worm's 1,092
cells disappear during development. In 1986 he published a paper
identifying two genes, ced-3 and ced-4, involved in their
disappearance. Then, in 1992, he found a third apoptosis gene, ced-9,
which was nearly identical to bcl-2, a human gene implicated in
lymphatic cancer. At that point, many biologists sat up and took
notice of apoptosis. Clearly, if a gene controlling apoptosis in a
worm had persisted through the eons of evolution to humans, then
apoptosis must be a universally important biological function. Columbia's
Dr. Taka-Aki Sato and Dr. Thomas Franke have helped biologists
understand pathways that transmit apoptotic signals from a receptor on
the cell surface usually to the mitochondrion. The mitochondrion
releases cytochrome c, which leads to the activation of caspases, the
enzymatic executioners. The caspases, about a dozen of which have so
far been identified, chew up the cell's DNA, destroy molecules that
attach a cell to its neighbors, and dismantle structural proteins
within the cell. |
||
Taka-Aki
Sato
Dr.Taka-Aki
Sato, associate professor of molecular oncology (in otolaryngology/head
& neck surgery and pathology), did much of his early work on the
bcl-2 family of genes. The bcl-2 protein plays a central role in
apoptosis. It sits on the outer membrane of the mitochondrion and
inhibits apoptosis. Several closely related proteins, such as BAD, Bax,
and bcl-xl, interact with bcl-2 and each other to either promote or
inhibit apoptosis. Dr. Sato, in collaboration with Dr. John Reed at
the Burnham Institute, identified, among other genes, BAG-1, a bcl-2
family member that inhibits apoptosis. Several
years ago Dr. Sato made the surprising discovery that yeast can serve
as a powerful model for studying apoptosis. A single-celled organism
would not be likely to have a suicide program, and yeast does not. But
when two genes, antiapoptotic bcl-2 and its proapoptotic cousin Bax,
are transferred into yeast, they establish a primitive form of the
death machinery. This
allowed Dr. Sato to use a clever system, known as the yeast two-hybrid
system, to find several other genes involved in apoptosis. When Dr.
Sato has one protein in a pathway and wants to know what it interacts
with, he attaches half the amino acids of a transcription factor to
the protein he already knows. He then attaches the other half of the
transcription factor to proteins that might interact with the original
protein. If two proteins bind in their normal interaction, the two
halves of the transcription also join together. The unified
transcription factor turns on a marker gene, beta-galactosidase, which
turns the yeast blue. "Using
a yeast system is a very powerful way to pull out proteins," says
Dr. Sato. P75NTR
is a neural receptor whose stimulation can either induce apoptosis or
prevent it, depending upon the tissue in which it is expressed. Dr.
Sato recently discovered the gene for the first protein known to
interact with P75NTR. The gene, which Dr. Sato named NADE, promotes
apoptosis. Dr.
Sato hopes eventually to induce apoptosis in cancer cells. Early in
his career, he worked for a company trying to develop Interluekin-2 as
a cure for cancer. Interleukin-2 is a growth hormone-like substance
that binds to receptors on cell surfaces. But interleukin-2's actions
were non-specific, causing both positive and negative effects,
depending upon the type of cell. Dr. Sato believed that the various
apoptosis pathways offered more specific and promising targets for
therapy. "I
thought apoptosis offered the fastest way to make a new drug against
cancer," says Dr. Sato. He
has been able to induce apoptosis in colon cancer cells by injecting
the cells with a tiny molecule that binds to FAP-1, a protein he
discovered in the Fas signaling pathway. By binding to FAP-1, the
tripeptide molecule kills colon-cancer cells by turning on their
apoptotic machinery. |
||
|
Thomas
Franke and the Akt signaling pathway
Dr.
Thomas Franke, assistant professor of pharmacology, has focused most
of his work in apoptosis on the Akt pathway, so named for one of its
central characters. Originally discovered as a viral oncogene, Dr.
Franke cloned and characterized the cellular Akt gene in mice in 1993.
Although he knew that the human homologue is overexpressed in some
forms of ovarian cancer, it was considered an "orphan"
because no one knew what function the Akt protein served or what
molecules it interacts with. In 1995, Dr. Franke linked Akt to a
growth-factor receptor, PDGF, and an important enzyme, PI3-kinase,
which regulates numerous physiological processes. In 1997, Dr. Franke
outlined the explicit biochemical steps leading from growth-factor
receptors through PI3-kinase to the activation of Akt protein and
showed that the pathway controlled cell survival in neurons and
hematopoietic cells. "The
. orphan. kinase has now moved to center stage as a crucial regulator
of life and death decisions emanating from the cell membrane,"
wrote Dr. Brian A. Hemmings in a commentary accompanying two papers in
Science. Since
joining the P&S faculty in August 1997, Dr. Franke has helped
identify many of the downstream targets of Akt, of which about a dozen
have been identified. Last November he helped show that Akt
inactivates caspase 9, the "effector" caspase that activates
other caspases in the final march to death. In March he helped show
that Akt promotes the production of nitric oxide in blood vessels. And
in April he contributed to a paper showing that calcium influx induces
apoptosis by triggering phosphorylation of BAD, a downstream target of
Akt. "It
has been wonderful to see how this pathway has fallen into place,"
says Dr. Franke. Dr.
Franke is now turning his attention to the role of Akt in cancer and
heart disease. He is studying how the expression of caspase 9 is
altered in patients with ovarian cancer. He is also beginning a study
of the role Akt plays in prevention of apoptosis in cardiac myocytes. "You
want your research to have human implications," says Dr. Franke.
"Columbia is a very good environment for connecting basic
research to clinical research." The
signaling pathways elucidated by Drs. Sato and Franke trigger
apoptosis in many cells. But the biological role that apoptosis plays
and the signals that trigger it vary from tissue to tissue. Several
P&S researchers have studied the biology of apoptosis in varied
tissues ranging from lymph nodes to neurons and the prostate. |
||
|
Seth
Lederman and B-cell selection
Dr.
Seth Lederman, associate professor of medicine, showed how apoptosis
helps select B cells that make infection-fighting antibodies. Each B
cell is capable of making a unique antibody. As with many other cell
types, apoptosis is the default program for B cells; they commit
suicide before maturing unless they receive a signal that saves them.
Helper T cells deliver the signal that saves B cells from apoptosis.
Dr. Lederman described the molecules on the surfaces of B cells and T
cells, known as CD40 and CD40-ligand, respectively, that interact to
save B cells. "Our
studies showed how the antibody response develops through apoptosis
and the rescue of selected cells," says Dr. Lederman. The
selection of B cells occurs in the germinal centers of lymph nodes. In
those centers dendritic cells release a limited number of antigens,
which are fragments of the invading organism. B cells compete to bind
with the antigens. The B cells whose antibodies bind most strongly to
the antigen, consume it, and present it on their surface. Helper T
cells bind to the B cell presenting those antigens and rescue it from
death. The saved B cell then begins to proliferate. But
the antibody genes in B cells mutate frequently. As a result, many of
the daughter cells produce antibodies slightly different from the
parent B cell. The daughter cells once again compete to bind the
antigen. The one making the antibody with the best fit, slightly
better than the parent cell, is saved by the T cell. The B cells go
through several rounds of this accelerated evolutionary process in
which the "fittest" B cells survive to produce another
generation of B cells. It eventually produces a B cell whose
antibodies bind tightly to the antigen and neutralize the foreign
body. At that point, it is released from the germinal center and
begins producing massive numbers of antibodies. "The
process of selecting B cells and refining the structure of their
antibody molecules is iterative," says Dr. Lederman. "Like
other forms of evolution, it tinkers with the antibody until the
structure is best." Dr.
Lederman believes apoptosis has transformed the field of physiology.
Physiologists used to define homeostasis largely in terms of the
plasma levels of various molecules, such as electrolytes or hormones.
Now they take a more cellular approach. "Apoptosis has focused
physiologists. attention on homeostatic mechanisms that balance cell
division and death." |
||
|
Ralph
Buttyan: apoptosis in prostate cancer
Dr.
Ralph Buttyan, associate professor of pathology (in urology), was
probably the first person at P&S to study apoptosis, although he
didn. t recognize its signature when he first observed it in 1984.
Apoptosis was still an obscure subject at that time, but it was well
known that prostate cells require the male steroid hormones known as
androgens to survive. That is why various forms of castration therapy
are used to treat advanced prostate cancers. When
Dr. Buttyan castrated rats with prostate cancer, most of the prostate
cells did dutifully die. But Dr. Buttyan made the enigmatic
observation that the prostate cells were becoming more active just
before their demise, synthesizing new gene products that are often
made by cells in the process of division. "This
was such an unexpected observation," says Dr. Buttyan 15 years
later. "That's why I decided to pursue it." A
short time later, Dr. Buttyan came across the literature of Kerr and
his colleagues describing apoptosis, which helped him understand what
was occurring in those prostate cells and led to the publication of
his findings in 1988. Several of the gene products he saw in the dying
prostate cells, such as c-myc, c-fos, and p53, have since been proved
to participate in apoptotic signaling and regulation. As Dr. Buttyan
and others have since discovered, apoptosis is closely linked to
mitosis.
"Proliferation
is a good time to weed out bad cells," says Dr. Buttyan. "It
presents the perfect opportunity for a cell to recognize internal
genetic problems so that they can be eliminated before they are passed
on to progenitors." Dr.
Buttyan has continued to study the role of apoptosis in the prostate,
especially in prostate cancers. His recent studies have shown that
castration induces apoptosis in endothelial cells lining the blood
vessels of the prostate. When those cells die, the blood supply to the
cancerous tissue is disrupted, depriving other cells in the prostate
of oxygen. While oxygen deprivation eventually causes such damage that
cells undergo necrosis, it appears that the prostate cells sense this
impending disaster and trigger their own suicidal program, thus
removing those cells in a more controlled and cleaner process. "I
now tell urologists, . In your use of castration therapy for prostate
cancer, you have been using antiangiogenic ther-apy for 50 years,.
" says Dr. Buttyan. Antiangiogenic therapy, in which tumors are
starved of their blood supply, has received attention recently as a
promising anticancer therapy. Some
prostate cancer cells inevitably survive castration. Dr. Buttyan has
shown that these cells express high levels of the antiapoptotic
protein bcl-2, making them resistant not only to castration but also
to other important cancer therapies. Dr. Buttyan and associate
research scientist Thambi Dorai have developed an antisense ribozyme
that degrades bcl-2 messenger RNA in cells and destroys the ability of
prostate cancer cells to make bcl-2 protein. They are now testing the
approach in animal models and believe that it holds promise as a
treatment for prostate cancer cells that resist other forms of
therapy. Dr.
Bernard Weinstein, the Frode Jensen Professor of Medicine, says the
concept of apoptosis has completely transformed the study of cancer.
Until recently, cancer was viewed as a disease that results from the
uncontrolled proliferation of cells. But today, it has become clear
that the failure of cells to die at the appropriate time is just as
important in the development of cancer. "Apoptosis
is a dominant theme in cancer research," says Dr. Weinstein.
"The concept has provided insight into why drugs work or don. t
work and has opened up whole new lines of research. Investigators are
looking for new forms of cancer therapy by screening for drugs that
cause apoptosis." |
||
Lloyd
Greene and neuronal survival
Dr.
Lloyd Greene, professor of pathology (in the Center for Neurobiology
and Behavior), has for many years studied the growth and
differentiation of neurons. His primary tool has been a cell line,
PC12, that he and Dr. Arthur Tischler developed in 1975. (Dr. Greene
jokes that some of the PC12 cells in his laboratory are older than
some of the people working there.) PC12 cells, originally derived from
tumors in the adrenal glands of rats, develop into cells resembling
sympathetic neurons when exposed to nerve growth factor (NGF). They
are one of the few models of neuronal cells and are widely used in
neuronal research. In
the early 1990s Dr. Greene became interested in how NGF promotes cell
survival. One of his students, Anna Batistatou, suggested that
depriving neurons of NGF might trigger apoptosis. Although researchers
at a recent conference reached a consensus that apoptosis probably
does not occur in neurons, Dr. Greene agreed to look for it anyway.
Dr. Green and Ms. Batistatou became one of the first groups to show
that neurons do indeed undergo apoptosis when deprived of growth
factors. In
subsequent years, Dr. Greene, working with Drs. Carol Troy, Leonidas
Stefanis, and Michael Shelanski, has described many features of
neuronal apoptosis. The researchers learned that removal of NGF
initiates changes in the expression and activity of molecules
associated with the cell cycle. Mature neurons don't normally divide. "When
you turn on the cell cycle in neurons, then they are in trouble and
can die," says Dr. Greene. But
Dr. Greene and his colleagues have uncovered evidence that the signal
turning on the cell-cycle genes cannot initiate apoptosis by itself.
It appears that a second signaling pathway, one normally associated
with stress, also must be stimulated for neurons to undergo apoptosis.
Dr. Greene believes neurons may need two independent signals to begin
apoptosis because they are so important to an organism's survival and
quite difficult to replace. "You
don. t want neurons to make a mistake and die if they don. t have
to," says Dr. Greene. His "two-key" hypothesis equates
neuronal apoptosis to nuclear missiles; both require two independent
signals for their launch. Dr.
Greene has turned his attention to the mitochondrion and the changes
it undergoes before releasing cytochrome c. He is using a powerful
technique developed by cancer researcher Bert Vogelstein to determine
not only genes newly expressed during apoptosis, but also changing
levels of gene expression. This technique, called SAGE, requires
high-throughput sequencing of genes. To that end, Drs. Greene and
Shelanski have purchased an AB prism 3700, the fastest gene sequencer
available and the first one in New York City. Dr.
Greene says he is most surprised by the fact that the three genes in
C. elegans originally discovered by Robert Horvitz describe the basic
process of apoptosis in higher animals. One gene codes for the enzyme
that cleaves the cell's proteins. One codes for a protein that
activates that enzyme, and another codes for a protein that inhibits
apoptosis. "In
mammalian cells, it has become much more baroque," says Dr.
Greene. "At the beginning I thought it would be a switch. You
turn it on and the cell dies. It's not that easy. The more I think and
read about this the more complex it gets. It's getting embarrassingly
complex." |
||
Robert
Burke and Parkinson's disease
Neurons
undergo apoptosis in several neurodegenerative diseases, including
Alzheimer' s disease, Huntington's disease, and amyotrophic lateral
sclerosis. But the exact role of apoptosis is unclear. "Is
it part of the disease? Can you stop it and make cells survive? Or is
the neuron so damaged that it must be removed?" asks Dr.
Shelanski. Dr.
Robert Burke, professor of neurology (in pathology), has focused on
unraveling the role of apoptosis in Parkinson's disease. In that
movement disorder, dopamine-emitting neurons in the substantia nigra
region of the brain die prematurely. In the early 1990s Dr. Burke
discovered that the dopamine neurons undergo apoptosis during
development if the target neurons are destroyed. The target neurons
are believed to provide growth factors to prevent the default
apoptosis. Dr. Burke has also shown that dopamine neurons undergo
apoptosis in a rat model of Parkinson's disease. "There's
no question that apoptosis occurs in dopamine neurons," says Dr.
Burke. The
key will be showing definitively that the dopamine neurons in the
substantia nigra undergo apoptosis in the brains of Parkinson's
patients. But it can be very hard to spot since apoptosis occurs
rapidly and leaves no trace. Dr. Burke has discovered that those
neurons undergoing apoptosis in rats express the caspase 3 gene. He
plans to search for expression of that gene in the brains of Parkinson's
patients who have died. |
||
Beth
Levine and apoptosis in viruses
Apoptosis
plays a role in more than just the proper development and protection
of a multicellular organism. It is a weapon in the conflict between
species. When a cell becomes infected by a virus, it often undergoes
apoptosis. "The
cell commits this altruistic suicide for the good of the organism,"
says Dr. Beth Levine, assistant professor of medicine. "Apoptosis
may have evolved as a defense mechanism." But
in the arms race of interspecies conflict, organisms often evolve new
ways to overcome the defenses. Viruses want a cell to survive long
enough for the viruses inside to reproduce many times. Then the cell
lyses, releasing the new viruses to infect other cells. "Viruses
want to keep a cell alive. They have evolved a number of defenses to
block apoptosis," says Dr. Levine. One of the first people to
study apoptosis in connection with viruses, Dr. Levine explains that
many viruses have picked up antiapoptotic genes from the cells they
infect. For example, in 1997 Dr. Sato and Dr. Yuan Chang, associate
professor of pathology, showed that the Kaposi's sarcoma-associated
herpesvirus expresses a bcl-2 gene that prevents infected cells from
committing suicide. Most
of Dr. Levine's work has focused on the Sindbis virus, one of a family
of RNA viruses that can cause acute encephalitis. The Sindbis virus is
an evolutionarily simple virus with such a tiny genome that it has no
gene to prevent apoptosis. But it has developed its own strategy for
avoiding removal by apoptosis. "Sindbis
virus preferentially infects neurons, which are more resistant to
virus-induced apoptosis," says Dr. Levine. Most
virally infected cells present a signal on their surface that tells
killer T cells to destroy them. But neurons do not. As a result the
immune system can. t kill the infected neurons, allowing the virus to
persist in those cells. Dr. Levine showed that the Sindbis virus,
previously believed to cause only acute infections, persists
indefinitely in neurons. She believes other viruses also may establish
persistent infections within neurons. "Our neurons are probably
loaded with viruses," says Dr. Levine. Dr.
Levine also showed that bcl-2 protects neurons from the apoptosis that
occurs when Sindbis infects other cell types. Bcl-2 also slows the
replication of the Sindbis virus inside neurons. Recently Dr. Levine
discovered a new gene, beclin 1, whose protein product interacts with
bcl-2 to protect neurons against apoptosis. Counterintuitively, it
also appears to play a role in tumor suppression and is mutated in
several breast-cancer cell lines. Her most recent work has identified
beclin 1 as the first mammalian gene known to control the process of
autophagy. "Autophagy
is the process by which cells degrade their own cytoplasmic contents,"
says Dr. Levine. "That process is defective in tumor cells, which
raises the interesting possibility that there are genetic links
between the regulation of apoptosis, tumor suppression, and autophagy." |
||
Future
directions and therapy
Many
more people at P&S are studying apoptosis in a variety of contexts.
Among them are Dr. Jean Gautier, assistant professor of genetics and
development (in dermatology), who has described apoptosis in the
earliest stages of development in the frog, Xenopus. Dr. Ramon Parsons,
assistant professor of pathology, has shown the tumor suppressor he
co-discovered, PTEN, is an antiapoptic element in the Akt signaling
pathway. Dr. Steven Moss, assistant professor of medicine, has shown
that Helicobacter pylori, the bacterium associated with ulcers and
other gastrointestinal disorders, induces apoptosis in the gut. Dr.
Abraham Spector, the Malcolm P. Aldrich Research Professor of
Ophthalmology, has shown that apoptosis contributes to the development
of cataracts in the eyes. P&S researchers also have seen evidence
of apoptosis in stroke, heart attacks, and diseases of the kidney. It
is also becoming clear that several anticancer therapies work by
promoting apoptosis. DNA damage caused by radiation causes apoptosis.
Several established chemotherapies, such as taxol, also have been
shown to induce apoptosis. New
therapies developed as a result of new knowledge about apoptosis are
mostly in the preclinical stages. Several researchers have shown
promising results in cell-culture and animal studies. The drug
sulindac sulfone, a derivative of the non-steroidal anti-inflammatory
drug sulindac, is being tested in clinical trials as a therapy against
prostate cancer. Dr. Weinstein first showed that the drug and related
compounds induce apoptosis in human prostate cancer cells in culture.
This occurs even if the cancer cells lack the important tumor
suppressor gene p53, have increased expression of bcl-2, or have
become androgen-independent. Based on those findings, Dr. Erik
Goluboff, assistant professor of urology, has begun a Phase III trial
of sulindac sulfone on 90 patients whose prostates have been removed
but whose rising levels of prostate-specific antigen indicate that the
cancer has not been vanquished. Apoptosis
has brought a certain balance to biology. Scientists are now learning
as much about the end of a cell's life as they already know about its
beginning. And in its complexity they see opportunities for therapy.
They realize that a better understanding of death may one day save
lives. |