Lessico
Arsenico

La parola
arsenico è un prestito dal persiano zarniq che vuol dire
"ornamento giallo"; zarniq venne adottato nel greco antico
nella forma arsenikón. L'arsenico era dunque conosciuto e utilizzato
in Persia e in altri luoghi fin dai tempi antichi. Poiché i sintomi
dell'avvelenamento da arsenico erano mal definiti, veniva usato spesso per
omicidi, fino alla scoperta del test di Marsh, un test di laboratorio molto
sensibile in grado di rivelarne la presenza nei tessuti. Un esempio
di mitridatismo![]() è la cosiddetta arseniofagia, che si osserva in individui i quali sono
abituati a tollerare senza alcun danno dosi di 25-30 centigrammi di arsenico
al giorno introdotti per via orale.
è la cosiddetta arseniofagia, che si osserva in individui i quali sono
abituati a tollerare senza alcun danno dosi di 25-30 centigrammi di arsenico
al giorno introdotti per via orale.
Durante
l'Età del Bronzo l'arsenico veniva spesso incluso nella lega di bronzo,
principalmente come impurità, il che rendeva il bronzo più resistente. Si
pensa che sia stato Alberto Magno![]() nel 1250
il primo a isolare l'arsenico elementare. Nel 1649 Johann Schroeder pubblicò
due diversi modi per preparare arsenico. Nell'età vittoriana l'arsenico
veniva usato come cosmetico per migliorare la carnagione e l'aspetto del volto
(il cosiddetto "pallore da arsenico"). Questo particolare veleno
venne anche usato per uccidere Rasputin, anche se questi ne prendeva tre gocce
ogni mattina, proprio per scongiurare un attentato del genere. I nobili della
congiura dovettero:sparargli, accoltellarlo, avvelenarlo e infine buttarlo,
sembra ancora vivo nonostante tutto, nel fiume Nieva.
nel 1250
il primo a isolare l'arsenico elementare. Nel 1649 Johann Schroeder pubblicò
due diversi modi per preparare arsenico. Nell'età vittoriana l'arsenico
veniva usato come cosmetico per migliorare la carnagione e l'aspetto del volto
(il cosiddetto "pallore da arsenico"). Questo particolare veleno
venne anche usato per uccidere Rasputin, anche se questi ne prendeva tre gocce
ogni mattina, proprio per scongiurare un attentato del genere. I nobili della
congiura dovettero:sparargli, accoltellarlo, avvelenarlo e infine buttarlo,
sembra ancora vivo nonostante tutto, nel fiume Nieva.
L'arsenico è l'elemento chimico di numero atomico 33. Il suo simbolo è As. È un noto veleno e un metalloide che si presenta in tre forme allotropiche diverse: gialla, nera e grigia. L'arsenico e i suoi composti trovano impiego come pesticidi, erbicidi e insetticidi. È inoltre usato in alcune leghe.
Dal punto di vista chimico, l'arsenico è molto simile al suo omologo, il fosforo, al punto che lo sostituisce parzialmente in alcune reazioni biochimiche, da cui il suo effetto tossico. Scaldato, si ossida rapidamente a ossido arsenoso, dal tipico odore agliaceo. L'arsenico e alcuni suoi composti sublimano, passando direttamente dalla fase solida a quella gassosa.
L'arsenico elementare si trova in due diverse forme solide, gialla e grigia metallica, le cui densità relative sono rispettivamente 1,97 e 5,73.
L'arseniato di piombo è stato usato fino a buona parte del XX secolo come pesticida sugli alberi da frutto, con gravi danni neurologici per i lavoratori che lo spargevano sulle colture e ci sono resoconti sull'uso di arseniato di rame nel XIX secolo come colorante per dolciumi.
L'applicazione di maggiore pericolo per il grande pubblico è probabilmente quella del legno trattato con arsenocromato di rame ("CCA" o "Tanalith", e la maggior parte del vecchio legno "trattato a pressione"). Il legname CCA è ancora in circolazione e in uso in molti paesi, ed è stato usato in modo massiccio durante la prima metà del XX secolo per strutture portanti e rivestimenti esterni di edifici in legno, dove c'era il pericolo di marcescenza o di attacchi di insetti. Anche se questo tipo di trattamento del legno è stato proibito nella maggior parte delle nazioni dopo la comparsa di studi che dimostravano il lento rilascio di arsenico nel terreno circostante da parte del legno CCA, il rischio più grave è la combustione di legno CCA, che concentra i composti di arsenico nelle ceneri: ci sono stati casi di avvelenamento da arsenico di animali e di esseri umani per ingestione di ceneri di legno CCA (la dose letale per un uomo è di 20 grammi di cenere, circa un cucchiaio). Il legno CCA recuperato da costruzioni demolite continua tuttavia a essere bruciato, per ignoranza, in fuochi domestici o commerciali; lo smaltimento sicuro di legno CCA continua a essere poco praticato e ci sono preoccupazioni in alcune zone massicciamente edificate con legno trattato all'arsenico per la futura demolizione delle costruzioni.
L'arseniuro di gallio è un importante semiconduttore, usato nei circuiti integrati. I circuiti realizzati in arseniuro di gallio sono molto più veloci (e molto più costosi) di quelli realizzati in silicio. A differenza del silicio, possono essere utilizzati nei diodi laser e nei LED per convertire direttamente l'elettricità in luce.
Il triossido d'arsenico è stato impiegato per la cura della leucemia promielocitica acuta in pazienti resistenti alla terapia con l'acido trans-retinoico.
Il triossido d'arsenico è impiegato in Australia come agente per la disinfestazione delle case dalle termiti. È usato anche nella realizzazione di fuochi d'artificio.
C'è una massiccia epidemia di avvelenamento da arsenico in Bangladesh, dove si stima che circa 57 milioni di persone bevano acqua da pozzi con concentrazioni di arsenico al di sopra dei limiti massimi di 50 parti per miliardo stabiliti dall'organizzazione mondiale per la sanità; tale arsenico è di origine naturale, e viene rilasciato dai sedimenti nelle acque di falda a causa delle condizioni anossiche del sottosuolo. Queste acque sotterranee hanno cominciato a essere utilizzate dopo l'avvio da parte di organizzazioni non governative occidentali di un grande programma di pozzi per ricavare acqua potabile, in modo da evitare l'uso di acque di superficie contaminate da batteri; purtroppo i test sull'acqua di falda per l'arsenico non vennero effettuati. Si pensa che molti altri paesi del sudest asiatico, come Vietnam, Cambogia e Tibet, abbiano ambienti geologici sotterranei tali da provocare la stessa alta concentrazione di arsenico nelle acque sotterranee.
L'arsenopirite, nota anche come mispickel (FeSAs) è il più comune minerale di arsenico, da cui l'elemento si ricava per arrostimento: il calore fa sublimare l'arsenico, lasciando come residuo solido il solfuro ferroso. La società Rumianca, di Riccardo Gualino, nello stabilimento di Carrara Avenza fondò la sua fortuna commerciale nella lavorazione delle piriti arseniose, come precursori di prodotti da usare nell'industria bellica e negli antiparassitari.
I composti più importanti dell'arsenico sono l'arsenico bianco (il suo solfuro), il verde di Parigi (arsenato di calcio) e l'arsenato di piombo. Tutti sono stati usati in passato come insetticidi e veleni agricoli. L'arsenico può raramente trovarsi puro in natura, ma più spesso si trova associato a argento, cobalto, nichel, ferro, antimonio o zolfo.
Oltre alle forme inorganiche summenzionate, l'arsenico si può trovare in un certo numero di composti organici nell'ambiente: una volta entrato nella catena alimentare, l'arsenico viene progressivamente metabolizzato in forme meno tossiche con un processo di metilazione.
Il
mitridatismo deriva da Mitridate VI re del Ponto![]() , con allusione alla sua
presunta assuefazione ai veleni, acquisita con la progressiva assimilazione di
dosi crescenti di sostanze tossiche. Come testimoniato da Galeno
, con allusione alla sua
presunta assuefazione ai veleni, acquisita con la progressiva assimilazione di
dosi crescenti di sostanze tossiche. Come testimoniato da Galeno![]() nella sua opera De antidotis, escogitò un sistema per non essere
avvelenato dai suoi nemici. A quel tempo, infatti, i monarchi vivevano nel
continuo timore di cadere vittima del veleno e ordinavano ai medici di corte
di elaborare antidoti. Mitridate, invece, iniziò ad assumere dosi crescenti
di sostanze tossiche, contro le quali sviluppò assuefazione. Sembra che
quando, sconfitto da Pompeo Magno, decise di togliersi la vita, non poté
avvelenarsi perché ormai divenuto immune ai veleni e dovette perciò farsi
trafiggere dalla spada di un suo soldato, Bituito. Secondo Aulo Gellio
nella sua opera De antidotis, escogitò un sistema per non essere
avvelenato dai suoi nemici. A quel tempo, infatti, i monarchi vivevano nel
continuo timore di cadere vittima del veleno e ordinavano ai medici di corte
di elaborare antidoti. Mitridate, invece, iniziò ad assumere dosi crescenti
di sostanze tossiche, contro le quali sviluppò assuefazione. Sembra che
quando, sconfitto da Pompeo Magno, decise di togliersi la vita, non poté
avvelenarsi perché ormai divenuto immune ai veleni e dovette perciò farsi
trafiggere dalla spada di un suo soldato, Bituito. Secondo Aulo Gellio![]() (Noctes Atticae XVII,16), vista l'inefficacia dei veleni, si suicidò,
così come afferma Dione Cassio
(Noctes Atticae XVII,16), vista l'inefficacia dei veleni, si suicidò,
così come afferma Dione Cassio![]() .
.
Il mitridatismo è una rara forma di resistenza antitossica che si stabilisce in taluni individui in seguito all'assunzione ripetuta di veleni. Consiste nella desensibilizzazione di un organo all'effetto di tossici che agiscono specificamente su di esso; ciò vale anche quando l'organo è sede di accumulo, di assorbimento o di trasporto del tossico in questione. Un esempio di mitridatismo è la cosiddetta arseniofagia, che si osserva in individui i quali sono abituati a tollerare senza alcun danno dosi di 25-30 centigrammi di arsenico al giorno introdotti per via orale. In questi soggetti l'arsenico provoca un'irritazione cronica dell'intestino (colite arsenicale) che, una volta instaurata, assicura per lungo tempo un limitato assorbimento dell'arsenico, e quindi la resistenza dell'organismo a questo veleno.
Il fenomeno del mitridatismo è attualmente messo in discussione; ad esempio, si oppone ad esso il fatto che se esso avesse validità generale, non dovrebbero verificarsi casi di tossicità cronica; d’altra parte, esiste un processo di assuefazione a sostanze psicoattive come la morfina, la cui ripetuta assunzione sembra determinare una sempre minore risposta dell’organismo (e, dunque, la necessità di assumere dosi crescenti). In generale, pertanto, oggi si tende a considerare il fenomeno del mitridatismo limitatamente ad alcune sostanze.

Arsenic is a chemical element that has the symbol As and atomic number 33. Arsenic was first written about by Albertus Magnus (Germany) in 1250. Its Atomic Mass is 74.92. Its Ionic Charge is (3-) This is a notoriously poisonous metalloid that has many allotropic forms: yellow (molecular non-metallic) and several black and gray forms (metalloids) are a few that are seen. Three metalloidal forms of arsenic with different crystal structures are found free in nature (the minerals arsenic sensu stricto and the much rarer arsenolamprite and pararsenolamprite), but it is more commonly found as arsenide and arsenate compounds. Several hundred such mineral species are known. Arsenic and its compounds are used as pesticides, herbicides, insecticides and various alloys.
The most common oxidation states for arsenic are -3 (arsenides: usually alloy-like intermetallic compounds), +3 (arsenates (III) or arsenites, and most organoarsenic compounds), and +5 (arsenates (V): the most stable inorganic arsenic oxycompounds). Arsenic also bonds readily to itself, forming, for instance, As-As pairs in the red sulfide realgar. In the +3 oxidation state, the stereochemistry of arsenic is affected by possession of a lone pair of electrons.
Notable characteristics
Arsenic is very similar chemically to its predecessor, phosphorus. Like phosphorus, it forms colourless, odourless, crystalline oxides As2O3 and As2O5 which are hygroscopic and readily soluble in water to form acidic solutions. Arsenic (V) acid, like phosphoric acid, is a weak acid. Like phosphorus, arsenic forms an unstable, gaseous hydride: arsine (AsH3). The similarity is so great that arsenic will partly substitute for phosphorus in biochemical reactions and is thus poisonous. However, in subtoxic doses, soluble arsenic compounds act as stimulants, and were once popular in small doses as medicinals by people in the mid 18th century.
When heated in air it oxidizes to arsenic trioxide; the fumes from this reaction have an odor resembling garlic. This odor can be detected on striking arsenide minerals such as arsenopyrite with a hammer. Arsenic (and some arsenic compounds) sublimes upon heating at atmospheric pressure, converting directly to a gaseous form without an intervening liquid state. The liquid state appears at 20 atmospheres and above, which explains why the melting point is higher than the boiling point. Elemental arsenic is found in many solid forms: the yellow form is soft, waxy and unstable, and is made of tetrahedral As4 molecules similar to the molecules of white phosphorus. The gray, black or 'metallic' forms have somewhat layered crystal structures with bonds extending throughout the crystal. They are brittle semiconductors with a metallic luster. The density of the yellow form is 1.97 g/cm³; rhombohedral 'gray arsenic' is much denser with a density of 5.73 g/cm³; the other metalloidal forms are similarly dense.
Applications
Lead hydrogen arsenate has been used, well into the 20th century, as an insecticide on fruit trees (sometimes resulting in brain damage to those working the sprayers), and Scheele's Green (a copper arsenate) has even been recorded in the 19th century as a coloring agent in sweets. In the last half century, monosodium methyl arsenate (MSMA), a less toxic organic form of arsenic, has replaced lead arsenate's role in agriculture.
The application of most concern to the general public is probably that of wood which has been treated with chromated copper arsenate ("CCA", or "Tanalith", and the vast majority of older "pressure treated" wood). CCA timber is still in widespread use in many countries, and was heavily used during the latter half of the 20th century as a structural, and outdoor building material, where there was a risk of rot, or insect infestation in untreated timber. Although widespread bans followed the publication of studies which showed low-level leaching from in-situ timbers (such as children's playground equipment) into surrounding soil, the most serious[citation needed] risk is presented by the burning of CCA timber. Recent years have seen fatal animal poisonings, and serious human poisonings resulting from the ingestion - directly or indirectly - of wood ash from CCA timber (the lethal human dose is approximately 20 grams of ash). Scrap CCA construction timber continues to be widely burnt through ignorance, in both commercial and domestic fires. Protocols for safe disposal of CCA timber are still in place only patchily; there is concern in some quarters about the widespread landfill disposal of such timber.
During the 18th, 19th, and 20th centuries, a number of arsenic compounds have been used as medicines, including arsphenamine (by Paul Ehrlich) and arsenic trioxide (by Thomas Fowler). Arsphenamine as well as Neosalvarsan was indicated for syphilis and trypanosomiasis, but has been superseded by modern antibiotics. Arsenic trioxide has been used in a variety of ways over the past 200 years, but most commonly in the treatment of cancer. The US Food and Drug Administration in 2000 approved this compound for the treatment of patients with acute promyelocytic leukemia that is resistant to ATRA. It was also used as Fowler's solution in psoriasis.
Copper acetoarsenite was used as a green pigment known under many different names, including 'Paris Green' and 'Emerald Green'. It caused numerous arsenic poisonings.
Used in animal feed, particularly in the US as a method of disease prevention and growth stimulation. Gallium arsenide is an important semiconductor material, used in integrated circuits. Circuits made using the compound are much faster (but also much more expensive) than those made in silicon. Unlike silicon it is direct bandgap, and so can be used in laser diodes and LEDs to directly convert electricity into light. Also used in bronzing and pyrotechny.
Occupational Exposures
Exposure to higher-than-average levels of arsenic can occur in some occupations placing workers at risk. Industries that use inorganic arsenic and its compounds include wood preservation, glass production, nonferrous metal alloys, and electronic semiconductor manufacturing. Inorganic arsenic is also found in coke oven emissions associated with the smelter industry.
History
The word arsenic is borrowed from the Persian word Zarniq meaning "yellow orpiment". Zarniq was borrowed by Greek as arsenikón, which means masculine or potent. Arsenic has been known and used in Persia and elsewhere since ancient times. As the symptoms of arsenic poisoning were somewhat ill-defined, it was frequently used for murder until the advent of the Marsh test, a sensitive chemical test for its presence. (Another less sensitive but more general test is the Reinsch test.) Due to its use by the ruling class to murder one another and its potency and discreetness, arsenic has been called the Poison of Kings and the King of Poisons.
During the Bronze Age, arsenic was often included in bronze, which made the alloy harder (so-called "arsenical bronze"). Albertus Magnus (Albert the Great, 1193-1280) is believed to have been the first to isolate the element in 1250. In 1649 Johann Schröder published two ways of preparing arsenic.
In the Victorian era, 'arsenic' (colourless, crystalline, soluble 'white arsenic') was mixed with vinegar and chalk and eaten by women to improve the complexion of their faces, making their skin paler to show they did not work in the fields. Arsenic was also rubbed into the faces and arms of women to 'improve their complexion'. The accidental use of arsenic in the adulteration of foodstuffs led to the Bradford sweet poisoning in 1858, which resulted in approximately 20 deaths and 200 people taken ill with arsenic poisoning.
In 2005, China was the top producer of white arsenic with almost 50% world share followed by Chile and Peru, reports the British Geological Survey. Arsenopyrite also unofficially called mispickel (FeAsS) is the most common arsenic-bearing mineral. On roasting in air, the arsenic sublimes as arsenic (III) oxide leaving iron oxides.
The most important compounds of arsenic are arsenic (III) oxide, As2O3, ('white arsenic'), the yellow sulfide orpiment (As2S3) and red realgar (As4S4), Paris Green, calcium arsenate, and lead hydrogen arsenate. The latter three have been used as agricultural insecticides and poisons. Orpiment and realgar were formerly used as painting pigments, though they have fallen out of use due to their toxicity and reactivity. Although arsenic is sometimes found native in nature, its main economic source is the mineral arsenopyrite mentioned above; it is also found in arsenides of metals such as silver, cobalt (cobaltite: CoAsS and skutterudite: CoAs3) and nickel, as sulfides, and when oxidised as arsenate minerals such as mimetite, Pb5(AsO4)3Cl and erythrite, Co3(AsO4)2. 8H2O, and more rarely arsenites ('arsenite' = arsenate(III). In addition to the inorganic forms mentioned above, arsenic also occurs in various organic forms in the environment. Inorganic arsenic and its compounds, upon entering the food chain, are progressively metabolised to a less toxic form of arsenic through a process of methylation. For example certain molds produce significant amounts of trimethylarsine if inorganic arsenic is present. Nickernuts are said to contain arsenic. See also Arsenide minerals, Arsenate minerals.
Toxicity
Arsenic and many of its compounds are especially potent poisons. Arsenic disrupts ATP production through several mechanisms. At the level of the citric acid cycle, arsenic inhibits pyruvate dehydrogenase and by competing with phosphate it uncouples oxidative phosphorylation, thus inhibiting energy-linked reduction of NAD+, mitochondrial respiration, and ATP synthesis. Hydrogen peroxide production is also increased, which might form reactive oxygen species and oxidative stress. These metabolic interferences lead to death from multi-system organ failure (see arsenic poisoning) probably from necrotic cell death, not apoptosis. A post mortem reveals brick red colored mucosa, due to severe hemorrhage. Although arsenic causes toxicity, it can also play a protective role.
Elemental arsenic and arsenic compounds are classified as "toxic" and "dangerous for the environment" in the European Union under directive 67/548/EEC. The IARC recognizes arsenic and arsenic compounds as group 1 carcinogens, and the EU lists arsenic trioxide, arsenic pentoxide and arsenate salts as category 1 carcinogens.
Arsenic is known to cause arsenicosis due to its manifestation in drinking water, “the most common species being arsenate and arsenite. The ability of arsenic to undergo redox conversion between As(III) and As(V) makes its availability in the environment possible. According to Croal, Gralnick, Malasarn, and Newman, "[the] understanding [of] what stimulates As(III) oxidation and/or limits As(V) reduction is relevant for bioremediation of contaminated sites (Croal). The study of chemolithoautotrophic As(III) oxidizers and the heterotrophic As(V) reducers can help the understanding of the oxidation and/or reduction of arsenic."
Arsenic in drinking water
Arsenic contamination of groundwater has led to a massive epidemic of arsenic poisoning in Bangladesh and neighbouring countries. It is estimated that approximately 57 million people are drinking groundwater with arsenic concentrations elevated above the World Health Organization's standard of 10 parts per billion. The arsenic in the groundwater is of natural origin, and is released from the sediment into the groundwater due to the anoxic conditions of the subsurface. This groundwater began to be used after western NGOs instigated a massive tube well drinking-water program in the late twentieth century. This program was designed to prevent drinking of bacterially contaminated surface waters, but failed to test for arsenic in the groundwater. Many other countries and districts in South East Asia, such as Vietnam, Cambodia, and Tibet, China, are thought to have geological environments similarly conducive to generation of high-arsenic groundwaters. Arsenicosis was reported in Nakhon Si Thammarat, Thailand in 1987, and the dissolved arsenic in the Chao Phraya River is suspected of containing high levels of naturally occurring arsenic, but has not been a public health problem due to the use of bottled water.
The northern United States, including parts of Michigan, Wisconsin, Minnesota and the Dakotas are known to have significant concentrations of arsenic in ground water. Increased levels of skin cancer has been associated with arsenic exposure in Wisconsin, even at levels below the 10 part per billion drinking water standard.
Epidemiological evidence from Chile shows a dose dependent connection between chronic arsenic exposure and various forms of cancer, particularly when other risk factors, such as cigarette smoking, are present. These effects have been demonstrated to persist below 50 parts per billion.
A study of cancer rates in Taiwan suggested that significant increases in cancer mortality appear only at levels above 150 parts per billion.
Analyzing multiple epidemiological studies on inorganic arsenic exposure suggests a small but measurable risk increase for bladder cancer at 10 parts per billion. According to Peter Ravenscroft, of the Department of Geography at the University of Cambridge roughly 80 million people worldwide consume between 10 and 50 parts per billion arsenic in their drinking water. If they all consumed exactly 10 parts per billion arsenic in their drinking water, the previously cited multiple epidemiological study analysis would predict an additional 2,000 cases of bladder cancer alone. This represents a clear underestimate of the overall impact, since it does not include lung or skin cancer, and explicitly underestimates the exposure. Those exposed to levels of arsenic above the current WHO standard should weigh the costs and benefits of arsenic remmediation.
Arsenic can be removed from drinking water through coprecipitation of iron minerals by oxidation and filtering. When this treatment fails to produce acceptable results, adsorptive arsenic removal media may be utilized. Several adsorptive media systems have been approved for point of service use in a study funded by the United States Environmental Protection Agency (U.S.EPA) and the National Science Foundation (NSF).
Isotopes
Arsenic has been proposed as a "salting" material for nuclear weapons (cobalt is another, better-known salting material). A jacket of 75As, irradiated by the intense high-energy neutron flux from an exploding thermonuclear weapon, would transmute into the radioactive isotope 76As with a half-life of 1.0778 days and produce approximately 1.13 MeV of gamma radiation, significantly increasing the radioactivity of the weapon's fallout for several hours.[citation needed] Such a weapon is not known to have ever been built, tested, or used.
Arsenic
Contamination of Groundwater
in South and East Asian Countries
April 2005
The World Bank and Water and Sanitation Program have recently completed and launched their report Arsenic Contamination of Groundwater in South and East Asia: Towards a More Operational Response. It is the first comprehensive international study that examines operational responses to the issue of naturally occurring arsenic in groundwater of Asian countries.
The water supply sector has a specific role to play in arsenic mitigation. An important lesson learned over recent years has been that arsenic cannot be treated as an isolated issue, with distinct programs and approaches, but it has to be integrated into broader water supply sector policies and approaches.
In practice, this would imply:
• Routine arsenic testing in planned water supply interventions in those areas where arsenic is likely to occur (there is sufficient information now about geohydrology in the region to roughly predict which areas are at risk),
• Application of well-known demand-based techniques to solicit from communities what type of arsenic mitigation measures they would prefer (the water supply sector has moved away from the “top-down approach” to development and, for the sake of effectiveness and sustainability, the same move is needed in dealing with the arsenic challenge).
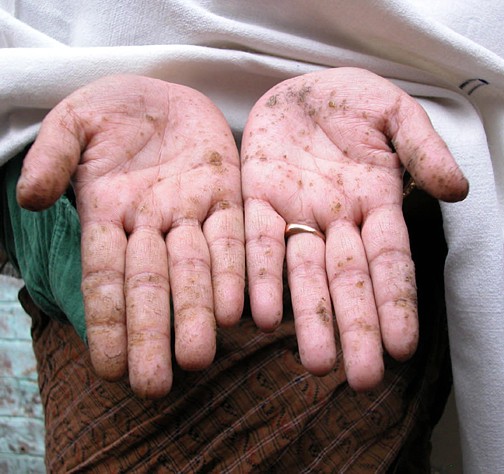
Signs of Arsenicosis: spots on the hands.
The solutions will take into account country and locality-specific characteristics and thus the approaches will vary. A range of tested options exist, from simple well-sharing in early phases, to provision of piped village water supply and to treatment of affected wells. The study outlines these options, and also analyzes them in economic, financial and social terms.
In summary, the study, which has drawn on information provided by a range of organizations – from governments to NGOs, donors, and academia to the World Bank’s own operations – shows that there is now enough information to act, and that actors should not be deterred by the complexity of the arsenic issue which is inevitably marked by a certain degree of uncertainty.
Much still remains to be done, however. The responses to arsenic contamination have so far lacked cohesion, both at national and global levels, and the problem needs to be addressed in a much more integrated and strategic manner. March 22nd marked the beginning of the UN Water for Life Decade and a more strategic approach to scaling up arsenic mitigation efforts would naturally be part of the Decade’s goals
http://web.worldbank.org